PEEK Is the Most Effective Alternative for the Medical Sector
Have you ever been tasked with selecting the most appropriate
Our implantable PEEK materials are widely used in spinal surgery, sports medicine, neurosurgery, cardiovascular and other implanted medical devices manufacture. The QMS was established to ensure a continuous and stable supply of excellent quality medical implanted materials.
Our medical implantable polyetheretherketone (PEEK) material, Brand Name: NATUREGEN®, has passed the biological compatibility test, conforming to the requirements of ASTM F2026 and ISO 10993.
The PEEK materials we provide include rods, granules, sheets and 3D printing filaments.


The NATUREGEN® PEEK Granule can be used for injection molding various implanted devices, especially for interference screws and suture anchors, with relatively few specifications and large quantities.

The NATUREGEN® PEEK Rod is suitable for machining, and the processing equipment can use conventional metal processing CNC equipment.

Φ12/16/20/25/30/35mm*1000mm,customization of other specifications is supported.
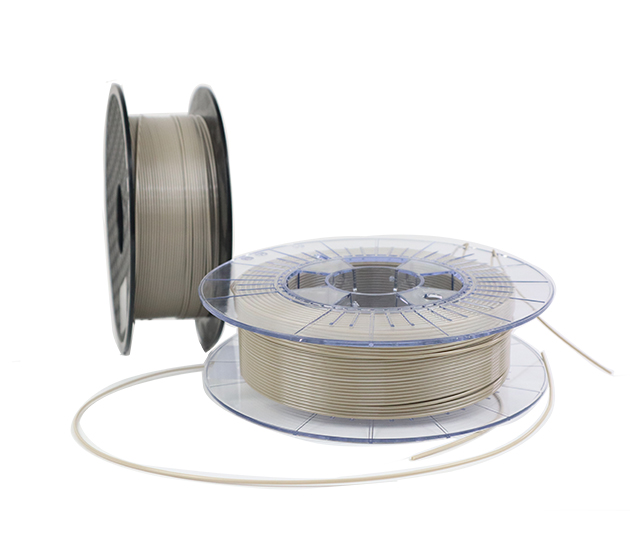
NATUREGEN® PEEK 3D printing filament is specially designed for FDM technology, with good melt fluidity, excellent mechanical properties, and biocompatibility, making it a popular choice for medical devices.

The PEEK sheet is tough, rigid, and quite strong, with good chemical and radiation resistance. Suitable for injection molding of implant products and various mechanical reinforcements.
Our production base in China has built a dust-free workshop in line with GMP requirements, with an annual capacity of 50 tons, which can meet the product needs of all kinds of customers. We are committed to the research and development of medical PEEK materials, with completely independent PEEK synthesis technology core patents, master implantation grade PEEK purification technology, and independent development of production equipment. In addition, we can provide consistent, stable performance, safe and reliable medical-grade materials to our customers.






We have completed the development and industrialization of medical implantable grade PEEK. NATUREGEN meets the requirements of ASTM F2026 and ISO 10993 series standards and has completed a series of biocompatibility testing programs. In addition, our PEEK materials are designed to meet the specific and stringent requirements of implantable medical devices.
With our expertise and advanced manufacturing technologies, and a diversified portfolio of strategic markets, we work closely with our customers and partners. As a provider of quality medical implant materials, JUNSUN implantable PEEK materials are widely used in spine surgery, sports medicine, neurosurgery, cardiovascular, and other implantable medical device manufacturing. No matter our customers’ needs, we can find the best solution to reduce production costs while improving sustainability.

Have you ever been tasked with selecting the most appropriate

In recent years, the medical industry has witnessed a surge
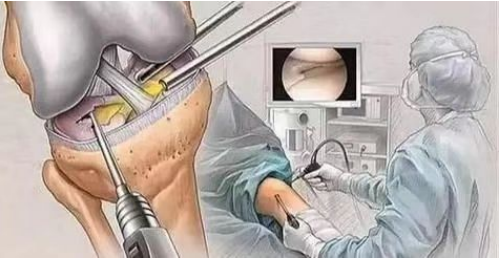

Cranioplasty refers to the operation of filling and repairing the

Junsun PEEK JS-S1 material conforms to the biological safety evaluation
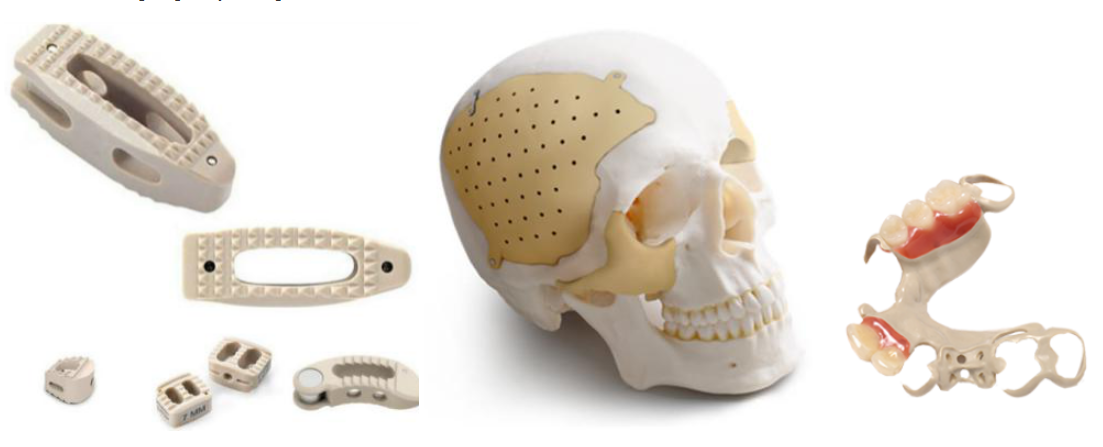
Polyether ether ketone (also known as PEEK) is a popular
Sign up our newsletter to get update news and article about company.
Copyright © 2022 JUNSUN, All rights reserved. Present by MoxCreative.